
GMP Dust
Quick Details Place of Origin: Zhejiang, China (Mainland) Brand Name: LTPM CHINA Model Number: GMP Cleanroom Item: Pharm
Description
Basic Info.
| Model NO. | Clean Room |
| Condition | New |
| Place of Origin | Zhejiang, China (Mainland) |
| Transport Package | Wooden Case |
| Trademark | LTPM |
| Origin | Zhejiang |
| Production Capacity | 25-100 Sets/Month |
Product Description

Quick Details
Place of Origin: Zhejiang, China (Mainland)
Brand Name: LTPM CHINA
Model Number: GMP Cleanroom
Item: Pharmaceutical Clean Room
Application: pharmaceutical,biological and cosmetic production
Service: Design, Supply, Installation and After sales Maitenance
Grade: 100,000 and 1,000,000 grade
Purification: Operator Purification, material purification and air purification
Size: depend on clients capacity
Material: GMP Cert Material
Roof: sandwich panels or corrugation steel plate
Wall Panel: all kinds of sandwich panels
Brand: LTPM CHINA
Packaging & Delivery
Packaging Details: | GMP Biological and Pharmaceutical Clean Room Materials and project packed by cases and wooden cases |
|---|---|
| Delivery Detail: | within 25days after receive your previous payment. |
GMP Biological and Pharmaceutical Clean Room
Service areas | biological and pharmaceutical products (oral agents, immune reagents, trauma supplies), pharmaceutical production (injection, tablet, capsule and drugs for external use only, traditional Chinese medicine), etc. |
Purifying engineering imaging: | |
Biological pharmaceutical purification workshop | 1. Operator purification a) Operator purification: biological medicine clean room (area) personnel purification program is as follows: b) Medicine clean zone shall be set up to the entrance of the gas chamber; The introduction of the gas chamber should be set interlock device. c) Air clean level the same sterile clean room and the sterile clean its personnel with room shall be installed separately purification. 2. material purification a) Medicine clean room raw and auxiliary materials, packaging material inward and outward, shall set up with material purification room. b) Into the sterile clean room raw materials, packaging material in addition to meeting the requirements above there should be set up in the entrance material room and the sterilization facilities. c) Material clean room or sterilization room and medicine clean room between shall set up gas chamber or pass ark. 3. 100,000 grade and above area should be in clean indoor wash clothes, dry, whole shall, when necessary, according to requirements sterilization. |
Simple Technical Data of Clean Room:
Project Sample:
Air cleanliness class | The maximum allowable number of suspended particles(pcs/ m2 ) | The maximum allowable number of suspended microorganisms | ||||
≥0.5um | ≥5um | Plank tonic bacteria(cfu/m2) | Settling microbe(cfu/DISH) | |||
100 | 3500 | 0 | 5 | 1 | ||
10000 | 350000 | 2000 | 100 | 3 | ||
100000 | 3500000 | 20000 | 500 | 10 | ||
Cleanliness level | DUST count/ m2 | Microbial number/ m2 | Ventilation rate/h | |||
≥0.5um | ≥5um | |||||
10 000 Class | ≤350 000 | ≤2 000 | ≤100 | ≥20 TIME | ||
100 000Class | ≤3500 000 | ≤20 000 | ≤500 | ≥15 TIME | ||

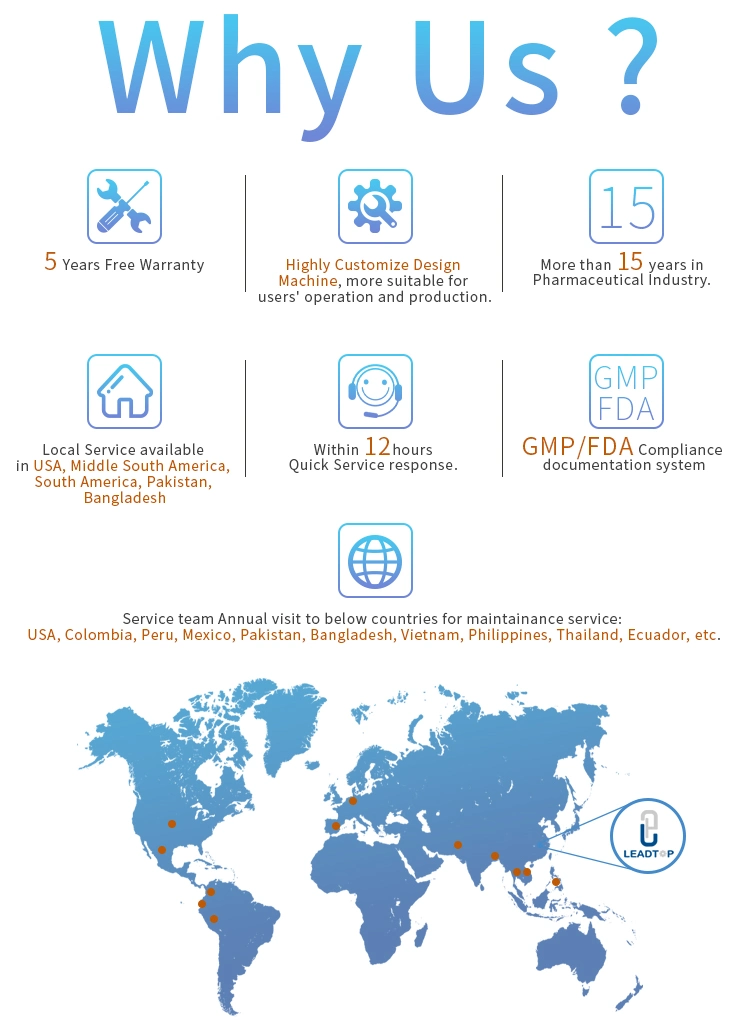


Q: What price terms you offer?
A: We can offer FOB, FCA, CFR, CIF, EXW and other price terms based on your request.
Q: What payment terms you take?
A: TT, LC, other terms are also workable.
Q: Will you help with installation and stuff training?
A: Yes, we can send our engineers to your place to guide the installation and train your workers if you need, but the buyer should
bear our technician's round tickets, accomodation, food and subsidiary USD100/day.
Q: How can I visit your factory?
A: Our factory is located in Ruian city Zhejiang Province. Just let us know your scheduel in advance and we will arrange
everything for you!
Any other doubts about our machine, please feel easy to contact with us.
Next: Double
Our Contact
Send now






